Mounting medium is usually very easily available commercially, but can a mounting medium be prepared at the lab? Are there different types of mounting mediums?

In this article I will discuss all these questions and describe how you can make a mounting medium yourself.
In general terms, microscope mounting medium can be made in one’s lab using glycerol as the base and a saline-based buffered solution like PBS which maintains the pH of the medium. Depending on planned use, additional ingredients may include antifade agents, adhesives, and anti quench agents. Let’s discuss in detail.
Let’s very briefly go over what a mounting medium is.
Mounting medium is a water-soluble or organic-solvent-based chemical that is used while preparing histological slides. Mounting is one of the last steps in the protocol of preparing a slide that is intended to be studied under the microscope. A mounting medium is used in this step over a sample already placed on the slide, and then a coverslip is put on top of it to make everything set in one place.
You can prepare your mounting mediums yourself using various ingredients. In fact, it is also believed that preparing the medium yourself sometimes gives better results than the commercially prepared ones. Most labs have their own recipe which they prepare using the ingredients of their choice and the purpose they have in mind— for example, whether they want to fix a slide temporarily or permanently, and the type of protein/tissue being examined. Therefore, the recipe usually varies from lab to lab.
As i stated earlier, the most commonly used mounting medium is composed of glycerol as the base and a saline-based buffered solution like PBS (phosphate-buffered saline) that maintains the pH of the medium. Other ingredients may include things like anti-fade agents (like 97% thiodiethanol or commercially prepared anti-fade kits), adhesives (epoxy resins, crystal bond thermoplastic resin, etc), anti-quench agents (free-radical scavengers; p-phenylenediamine, propyl gallate, etc), fluorophores (prevents photobleaching), and agents for ringing the coverslip like, glycerine (liquid), and vaseline or simple nail polish (solid).
How To Prepare A Mounting Medium?
Mounting medium is prepared using glycerol and PBS in the ratio of 9:1. Northern Arizona University says various studies have proved that the optimum pH of the medium should be maintained at 8.5 to 9.0, as at this pH the sample on the slide can be preserved for a longer duration of time.
A wide range of ingredients can be used to prepare a mounting medium, but the simplest recipe includes 3 ingredients: glycerol, an anti-fade agent, and a buffer. Here’s a recipe recommended by Northern Arizona University and the Harvard Medical School:
- Glycerol: 90 ml in 50-90% concentration. Glycerol raises the refractive index (RI) of the sample and helps in the formation of a brighter and higher resolution image. It is important to match the refractive index (RI) of the mounting medium to the RI of the sample. When the light passes from one medium to another, it bends (refraction). So, if the RI of both the mounting medium and the specimen is the same (or almost similar) the scattering of light would be less. Glycerol is used in the concentration of about 50-90% depending on which type of imaging is being done. Harvard Medical School suggests 90% glycerol should be used for fluorescence images, while 50% glycerol should be used for DIC images. This will give you the best image in the respective setting.
- Buffer: 10 ml 0.1M phosphate buffer, or 10 ml 0.1 M TRIS buffer.
- Anti-fade agent: p-phenylenediamine hydrochloride- 100 mg. This is considered to be the best anti-fade agent. But it is known to react with blue dyes and destroy it slowly, and so, over time the image may weaken. Therefore, this anti-fade agent is not recommended if you are planning to store your slide for a longer period. Also, it is toxic in nature. Wear all protective gear and avoid inhaling it. Its toxicity can cause serious organ damage and can even be fatal. Alternatively, you can use 0.5% n-propyl gallate– 500 mg. This agent can be used with living cells as well, and you should be able to store your sample for a longer time with this agent when compared to p-phenylenediamine hydrochloride. However, it interferes with the apoptosis of the cells, and therefore it may not be the most suitable anti-fade agent if you are preparing the medium to study biological processes. Other agents that can be used are ascorbic acid and 1,4 diazabicyclo (2,2,2) octane (DABCO).
- Mix these ingredients and shake well. You may need to warm it up to 37 degrees Celsius, especially if you are using n-propyl gallate as the anti-fade agent since it is not readily soluble. Heating it up will help with that. Alternatively, you can also leave it to dissolve slowly overnight.
- The mounting medium should then be stored at 4 degrees celsius and can be used for 1-2 weeks.
- An ideal mounting medium is a transparent one. So, if you notice a color change, discard it. Also, if you see some discoloration while preparing the medium, discard it, as that would mean some contamination has happened.
This recipe will help you form a temporary slide. Glycerol helps preserve the slide for 3 months at least. You can store it for a longer period if you store it at -20 degrees Celsius in the dark to prevent any kind of photobleaching.
To prepare a permanent slide, organic solvent-based chemicals containing xylene or toluene are used.
Why Is Mounting Medium Used?
Mounting medium has a few advantages:
- The most important role that any mounting medium plays in the maintenance of an appropriate refractive index (RI) for the formation of a good quality image under the microscope. The recommended RI of the mounting medium is around 1.53. The closer the value of RI of the tissue and the mounting medium to this value, the brighter and clearer the image would be. Glycerol does this job in a mounting medium, with an RI of 1.47.
- Secondly, the mounting medium fixes the specimen in one place and prevents it from drying out.
- It helps preserve the slide for a longer duration of time. A specimen is very finely sliced, and is, therefore, very delicate. By putting a mounting medium on top of it and then covering it with a coverslip protects the structures of the specimen. It also prevents moisture from coming in contact with the specimen which would promote growth of bacteria. Hence, it prevents its degradation by bacteria. Anti-fade agents protect the specimen from damage caused by exposure to light.
Types of Mounting Media
There are two types of mounting media.
- Organic Solvent Based Media: These are composed of natural or synthetic resins dissolved in solvents like toluene, benzene, xylene, etc. They are used when the sample needs to be dehydrated. They are also known as the adhesive medium; meaning it sticks the specimen to the slide and then it hardens which permanently adheres the coverslip on top of the sample. For this reason, permanent slides can be prepared using these. Some of the examples of a solvent-based media are Canada balsam, phenol balsam, Euparal, etc.
- Water-Based Media: These are composed of ingredients that are water-soluble in nature. These are easier to use and are less toxic. But they can’t be used to prepare permanent slides. They can be used to prepare temporary slides that last for a few months. Examples include glycerol-based media, Apathy’s medium, Farrant’s medium, etc.
A slide that is prepared using a liquid medium is known as a wet mount slide. But in some cases, like when spores and pollens are the study subject, no mounting medium is used. A coverslip is directly put over the specimen. In this case, the mounting medium is air, as air surrounds the specimen under the coverslip. This type of slide is known as a dry mount slide. But dry mount usually produces a poor quality image because the RI of air is 1.0, which is pretty far off from 1.53, and this promotes scattering of light to a great deal.
How To Use Mounting Medium?
Once the initial steps of the slide preparations are done— fixing, embedding, sectioning, staining, etc, the mounting medium is put on top of the specimen. Use 6-8 microL of this solution per 18 mm coverslip. Too much of the solution will spill out of the slide.
Glycerol stays in the liquid state, i.e. it never hardens. So, it’s essential that you seal the coverslip (especially if you are planning to keep the slide for a few months) so that the glycerol stays as it is, and there is no entry of moisture or contaminants inside the slide. Sealing the coverslip at its edges to achieve this is known as the ringing of the coverslip. To seal the coverslip a variety of agents can be used. For example, paraffin wax and vaseline— which will form a temporary slide.
A more permanent sealant can be formed using paraffin wax (10 parts) and colophonium resin (40 parts). This is known as the Du Boyer wax-colophonium resin mixture. This is a more permanent sealant. Lastly, clear nail polish can be used as a sealant too. It’s easily available and easy to use. Sealing with nail polish can preserve the slide for a few months. Asphalt-based cement is another option if you are going for a permanent slide.
If you are using a solvent-based medium, you might not need to apply a sealant as it usually contains natural or synthetic resin, which hardens, thereby preserving the specimen within it.
Make sure that the mounting medium you are using is transparent in color, and has a RI of 1.53. If you see any color change— which can range from yellowish, brownish, or black— discard the medium.
Here’s a video that will help you understand the process of using a mounting medium and making permanent slides:
Difference Between A Permanent And A Temporary Slide?
As the name suggests, a permanent slide is a slide that is prepared to be preserved for a very long period— years. Such slides when prepared well can even survive for a century. The permanent slides are prepared using the solvent-based medium, as they contain the resin component which eventually hardens and protects the specimen from damage.
The disadvantage of a permanent slide is that it cannot be used to study a live cell since the specimen is fixed on the slide.
On the other hand, a temporary slide is prepared using a liquid mounting medium like the one mentioned in this article. They can be used to prepare a temporary slide. As mentioned above, to increase its longevity, ringing of the coverslip can be done using a sealant.
The advantage of a temporary slide is that it can be used to study a living cell since the specimen stays in a liquid medium.
The Bottom Line
You can easily prepare a mounting medium using three simple ingredients. The recipe mentioned in this article is water-soluble, and therefore will be useful in preparing a temporary slide. Depending on your goals, you can choose other ingredients. Choosing a solvent-based medium will be ideal if you want to prepare a permanent slide.
Click here to read about the maximum magnification of a confocal microscope.
Zbrush Dynamesh not Working: Solutions
Zbrush in itself is an amazing tool for organic 3d modeling such as that used in medical animations. Dynamesh adds another level to the very unique and intuitive modeling workflow of the software. With it, you will benefit from efficiency in modeling and cut down on time and effort spent on your project.

However, dynamesh can sometimes be problematic. In this article I will help you solve the issue of dynamesh not working.
Continue reading “Zbrush Dynamesh not Working: Solutions”
Can you Minor in Medical Illustration?
Medical illustration is a growing interest of students from different educational backgrounds these days as the industry is growing at a good rate. What happens if you have some interest in medical illustration but are not sure you want it as your major?
In this article I will answer the question on the minds of many current and future university students, which is “Can you minor in medical illustration?”
Continue reading “Can you Minor in Medical Illustration?”
Maximum Magnification of a Confocal Microscope? Factors Involved
The confocal microscope is an amazing and useful piece of equipment, allowing one to look into tissues with greater clarity and without the limitations of other microscopes. Not only can it be used to exactly pinpoint your stained objects of interest, but the 3D stacks it is capable of producing can be turned into virtual 3D worlds and animations you can fly through as if you were inside the living tissue.

However, even the most knowledgeable science professionals sometimes miss out on the essential details. You could own a piece of certain laboratory equipment and remain unsure of its full potential. In this article I will discuss the maximum magnification of a confocal microscope and related items.
Continue reading “Maximum Magnification of a Confocal Microscope? Factors Involved”
How to Fix a Non Working Wacom Tablet?
Many of us who work with graphics including 3D are familiar with graphics tablets. Companies like Apple, Microsoft, Samsung, Wacom, and Huion sell different types of tablets according to the needs of the users. Wacom’s graphics tablets are favored by many due to their ease of use and all the functions.

However, no product is perfect, and these devices can run into problems. Let’s explore all the things you can do when your tablet stops working.
Continue reading “How to Fix a Non Working Wacom Tablet?”
How to do Deconvolution in ImageJ: The Easy Way
If you are having problems with blurring in your microscopy images, going through deconvolution is highly likely to improve your results.

Deconvolution is not as difficult as it sounds, you just need to understand a few basic concepts and know which software can perform the task. This article will answer such questions.
As a general rule, to perform deconvolution you use a calculated theoretical or extracted point spread function from your microscope, which you then apply in a deconvolution plugin to your image or image stack. The result which may take several iterations is a deconvolved image with less blur and more accurate object dimensions for data analysis.
But let’s start at the beginning by getting familiar with the terms and then describe the steps.
What is deconvolution?
Just to be on the same page if anyone is new to the term deconvolution, here are the basics needed to perform the process. Deconvolution is a computationally intensive process of clearing up a microscopy image which has undergone convolution or distortion due to the objective’s limited aperture. It makes the image appear closer to the actual real life subject being viewed. Deconvolution can involve increasing resolution, contrast, and deblurring.
Deconvolution can be used on images from various types of microscopes, including confocal, multi photon and light sheet. In addition, it can improve the quality of images which were affected by motion during capture. Deconvolution is performed on an entire Z series of images.

While it is said that the pinhole and nature of the confocal microscope eliminates the need for deconvolution later on by getting rid of the signal from other focal planes, this is often not the case. True deconvolution uses algorithms on existing images and can likely help with the quality of confocal photos. In confocal, deconvolution helps with elongation of spherical objects in the Z axis.
Deconvolution is important whenever image analysis will be involved (and in science that is most of the time). If not performed, the results of image analysis may contain a systematic error.
What is a Point Spread Function (PSF)
The Point Spread Function is a function or shape of your optical system which describes the convolution. It does so by describing the path light from a point source of light takes through the microscope. It has a different shape depending on the type of imaging used. The point spread function is much wider for air lenses and that is why they have lower resolution unlike oil immersion lenses.
If the point spread function can be measured, the image can be corrected. During convolution, the point spread function is applied to every point in the sample which as a result gives the final, altered image. Anotherwords the image you observe is a product of the point spread function times the real object. Deconvolution reverses this.
To perform deconvolution you need to generate the point spread function for your specific system. There are different ways to do this, discussed later.
Here is an online calculator if you are interested in calculating a PSF (not needed for the process of deconvolution itself).
2D or 3D Deconvolution?
Deconvolution can be performed on single images or 3D stacks (file with multiple images at different depths). 3D deconvolution is more computationally intensive. However, as with many procedures that take more time, the results are often more accurate. 3D deconvolution works through iterations with better quality images resulting from each subsequent cycle. Blur is treated on every layer available.
In terms of what you need for 2D vs 3D deconvolution, for a 3D stack you have to enter the Z spacing of your stack as well as the number of images in stack.
When you are acquiring your 3D stack, be sure to go beyond your object of interest so that deconvolution results are the best possible. This is because you want to cover the PSF of not only the subject of interest but also other points in the slide.
What you need for deconvolution
To perform deconvolution you need one of the following:
- image/stack of point like objects/beads taken on the same scope as your image of interest to extract the PSF via a plugin
- full specs of the imaging system/scope used including excitation/emission wavelength, refractive index of lens, and aperture to compute theoretical PSF via a plugin
Plugins for deconvolution
There are several programs and plugins out there specifically made for deconvolution which will make the process much easier. Some work with ImageJ, others with Matlab, and still others are stand alone Java programs. In certain cases, a single program can run on all of these. You can experiment with which ones work best for you.
Point Spread Function Plugins:
PSF Generator
Mentioned earlier, PSF Generator is a Java program that can be used to generate a point spread function. It has more than a dozen models and can work with ImageJ as a plugin.
Diffraction-PSF-3D plugin
Diffraction PSF 3D creates a theoretical z stack of the theoretical point spread function. All you need to do is enter data into various windows based on the raw image, including specs such as slice spacing, image pixel spacing, width, height, wavelength used, numerical aperture and index of refraction of your media.
Deconvolution Plugins:
Parallel Spectral Deconvolution
Parallel Spectral Deconvolution is an ImageJ plugin for deconvolution which takes advantage of higher spec hardware and multi core processors.
Deconvolution Lab
DeconvolutionLab is nicely laid out open source GUI plugin with various deconvolution algorithms including Naive Inverse Filter, Tikhnonov Inverse Filter, and Richardson-Lucy. It can be linked to ImageJ or Matlab and runs as a stand-alone.
Iterative Deconvolve 3D plugin
The Iterative Deconvolve 3D plugin is an ImageJ plugin for both 2D and 3D deconvolution. It is iterative and has a built in Wiener filter as a preliminary step. The plugin uses a point spread function image Z stack. It is an iterative plugin which works much better for noisy microscopy images.
Deconvolution in ImageJ
Deconvolution may be performed using ImageJ and associated plugins adequately depending on the condition of the source material and other factors. It is best to try it and see if it meets your expectations.
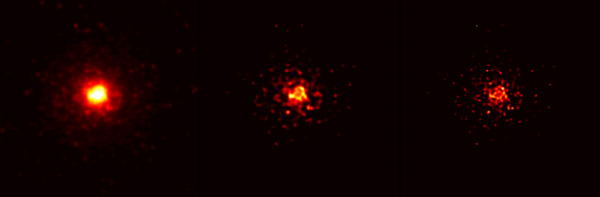
“File:Blind deconvolution illustration.png” by Huo Zhuoxi is licensed under CC BY-SA 3.0
Keep in mind that some plugins for deconvolution only work on grayscale images. If you are using one of these plugins, you need to go in ImageJ to Image/Type/8 bit to convert your image to grayscale. If you have a color image, you just have to split the color image into a layer for each color channel and then put it back together after the deconvolution is done.
This is easier than it sounds. With color image open, go to Image/Color/Split Channels, save the resulting images with names to remember which was which color, deconvolve them separately as described below, then go to Image/Color/Merge Channels.
Below are steps for performing deconvolution in ImageJ using theoretical PSF:
-Open your image dataset
-Go to plugins/3D/Diffraction PSF 3D
-Enter your microscope and specimen values to create PSF
-Go to plugins/Parallel Spectral Deconvolution and choose 3D or 2D
-Choose source image and created PSF file
-Select options such as algorithm (experiment with it) and click Deconvolve
-Compare deconvolved slice with original one
-Repeat deconvolution with different settings if necessary
Below are steps for performing deconvolution in ImageJ using PSF extracted from a stack:
-Open your stack of point like objects/beads
-Go to plugins/PSF Generator and run it
-Save PSF
-Open your image dataset
-Go to plugins/Parallel Spectral Deconvolution and choose 3D or 2D
-Choose source image and created PSF file
-Select options such as algorithm (experiment with it) and click Deconvolve
-Compare deconvolved slice with original one
-Repeat deconvolution with different settings if necessary
It is that simple. In addition to experimenting with the various algorithms, you can try different plugins and free vs commercial software packages if you are not happy with the results. So basically you can substitute plugins in the steps where plugins are mentioned to see what works best for you.
Deconvolution speed
Deconvolution can be very hardware intensive. The deconvolution process takes several iterations. You may need a high spec workstation and GPUs for certain stacks and deconvolution procedures. It also matters which software you are using. A GUI heavy program with easy to navigate graphics may not be your optimal solution, keep that in mind.
Below is a useful video on deconvolution from the Howard Hughes Institute which gives a more detailed explanation:
I hope this article made deconvolution easier to understand and took away any fear of the process.
Click the following link to learn how to improve MRI quality.
